
【Methods】 The discovery of a new antibody for BRIL-fused GPCR structure determination / BRIL融合GPCR構造決定のための新しい抗体の発見
Complete amino acid sequences and constructs for crystallization. /結晶化のためのコンストラクト
Culture and membrane preparation. /Sf9を用いたGPCRの培養
Production of monoclonal antibody fragment (SRP2070Fab). /マウス膵臓によるモノクローナル抗体フラグメント(SRP2070Fab)の作成
Purification of 5HT1B-BRIL/ERG/SRP2070Fab. /5HT1B-BRIL/ERG/SRP2070Fabの精製
Crystallization and data collection of 5HT1B-BRIL/ERG/SRP2070Fab complex./5HT1B-BRIL/ERG/SRP2070Fab複合体の結晶化と回析データ
Purification of AT2R-BRIL/s-Ang II/SRP2070Fab./AT2R-BRIL/s-Ang II/SRP2070Fabの精製
Crystallization and data collection of AT2R-BRIL/s-Ang II/SRP2070Fab complex. /AT2R-BRIL/s-Ang II/SRP2070Fab複合体の結晶化と回析データ
complete amino acid sequences and constructs for crystallization.
The additional N-terminal residue retained after cleavage with tobacco etch virus (TEV) is italicised.
The sequence of AT2R residues 35-346 (UniProt accession: P50052) with the BRIL insertion (underlined) is provided in Table 2.
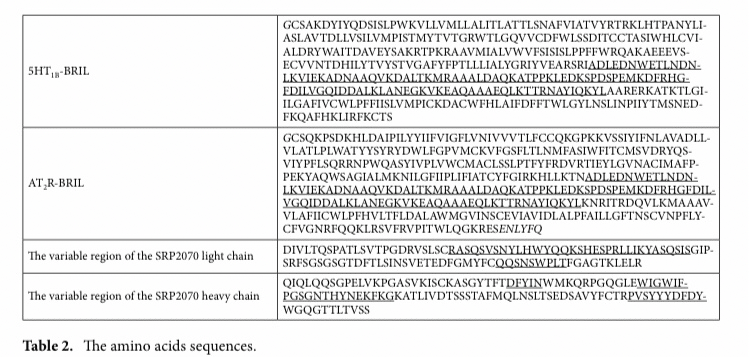
The mutations, L93V and F133W, were added to improve stabilisation.
The additional N- and C-terminal residues retained after cleavage with tobacco etch virus (TEV) cleavage are italicised.
The subclass of the SRP2070 was a mouse IgG2a.
The sequence of the variable region of the light chain is provided in Table 2; CDR-L1, CDR-L2, and CDR-L3 are underlined.
The sequence of the variable region of the heavy chain is provided in Table 2; CDR-H1, CDR-H2, and CDR- H3 are underlined.
Codon-optimised cDNA encoding H. sapiens AT2R-BRIL and 5HT1B-BRIL was cloned into pFastBac1 (Thermo Fisher Scientific, Waltham, MA, USA). For AT2R-BRIL, the sequences encoding haemagglutinin (HA) and FLAG tags followed by a TEV cleavage site were added to the N-terminus, and a TEV cleavage site followed by a His8 tag were added to the C-terminus.
For 5HT1B-BRIL, haemagglutinin (HA), FLAG, and His8 tags fol- lowed by a TEV cleavage site were added to the N-terminus.
culture and membrane preparation.
AT2R-BRIL was expressed in Spodoptera frugiperda (Sf9) cells using the baculovirus-based expression system (cell density=2.0–4.0×106 cells/mL; MOI=0.5; expression time = 60 h). The culture was harvested by centrifugation (7,000 × g, 10 min, 4 °C), and cell pellets were flash frozen at −80 °C. Frozen cells were thawed and suspended in the hypotonic buffer (10 mM HEPES pH 7.5, 20 mM KCl, 10 mM MgCl2, 1× protein inhibitor cocktail tablet).
The suspension was centrifuged, and the pellet was resuspended in high osmotic buffer [10 mM HEPES pH 7.5, 10 mM MgCl2, 20 mM KCl, 1 M NaCl, 1 × protease inhibitor cocktail (Sigma Aldrich, Saint Louis, MO, USA)].
The cells were lysed using a dounce homogeniser, and the lysate was centrifuged (100,000×g, 30 min, 4 °C).
The pellet was resuspended in high osmotic buffer containing 40% glycerol and stored at − 80 °C. Plasma membranes expressing 5HT1B-BRIL were prepared using the same method as for AT2R-BRIL.
Production of monoclonal antibody fragment (SRP2070Fab).
Purified GPCR-BRIL proteins were reconstituted into liposomes consisting of 20:1 (w/w) egg phosphatidylcholine (Sigma-Aldrich)/monophos- phoryl lipid A (Sigma-Aldrich).
MRL/lpr mice were immunised with liposomal GPCR-BRIL. After immunisa- tion, the mouse spleens were removed, and spleen cells were fused with myeloma cells.
Hybridoma cells were screened by liposome-ELISA, denatured dot blot analysis, and fluorescence size exclusion chromatography (FSEC).
To screen antibodies that specifically bind to the antigen with correct structure, but not the denatured one, liposome-ELISA positive and denatured dot blot clones were pooled, and then we confirmed the binding capacity in aqueous solution by FSEC.
Hybridoma cells producing SRP2070 were intraperitoneally administered to mice, and ascites were collected. SRP2070 was purified by ammonium sulphate precipitation and protein G column chromatography.
The subclass of SRP2070 was mouse IgG2a.
After cleaving SRP2070 with papain, SRP2070Fab was purified by protein A column chromatography to remove the Fc portion and further purified by size exclusion chromatography (Superdex200 10/300 GL, GE Healthcare) by the changing buffer into PBS.
Purification of 5HT1B-BRIL/ERG/SRP2070Fab.
The construct for structural analysis was expressed in accordance with a previous study17. The amino acids from Leu240 to Met305 were replaced with BRIL. In addi- tion, the glycosylation site was removed by deleting 32 amino acid residues at the N-terminus. The HA signal sequence, FLAG tag, His10-tag, and TEV protease recognition site were added. Furthermore, the L138W muta- tion was introduced to increase thermal stability. The DNA coding sequence was inserted into pFastBac1 using BamHI and HindIII.
Purified plasma membranes were thawed on ice and resuspended in solubilisation buffer (50 mM HEPES pH 7.5, 500 mM NaCl, 10% glycerol, 20 mM imidazole, 1% n-dodecyl-β-d-maltoside (DDM), 0.2% cholesteryl hemisuccinate (CHS), 1 × protein inhibitor cocktail) supplemented with 100 mg/mL iodoacetamide (Wako Pure Chemical Industries, Ltd.) and 50 μM ERG (Sigma Aldrich, Saint Louis, MO, USA), and then solubilised by rotat- ing gently for 3 h at 4 °C.
The supernatant was isolated by centrifugation at 235,000 × g for 45 min and incubated with TALON Superflow Metal Affinity Resin (Clontech; 1 mL of resin per 500 mL of original culture volume was used) for 2 h at 4 °C.
After incubation, the resin was washed with five column volumes of wash buffer (50 mM HEPES pH 7.5, 500 mM NaCl, 10% glycerol, 0.1% DDM, 0.02% CHS, 20 mM imidazole, 50 μM ERG, and 10 mM ATP), followed by five column volumes of wash buffer containing 800 mM NaCl.
5HT1B-BRIL protein was eluted with three column volumes of elution buffer (50 mM HEPES pH 7.5, 500 mM NaCl, 10% glycerol, 0.1% DDM, 0.02% CHS, 500 mM imidazole, and 50 μM ERG), concentrated, and then buffer exchanged into wash buffer without imidazole.
The sample was treated with His-tagged TEV protease (expressed in-house) at 4 °C overnight to remove the N-terminal tags. The His-tagged TEV protease and cleaved N-terminal fragment were removed using Ni–NTA fast flow resin (GE Healthcare).
5HT1B-BRIL/ERG complex was collected as the Ni–NTA column flow-through fraction and concentrated to ~ 30 mg/mL using a 100-kDa MWCO Amicon concentrator.
The 5HT1B-BRIL/ERG/SRP2070Fab complex was prepared by mixing 5HT1B-BRIL/ERG and SRP2070Fab at a molar ration of 1:1.5 for 1 h on ice.
The complex was purified by SEC (Superdex 200 increase 200, GE healthcare).
Peak fractions containing 5HT1B-BRIL/ERG/SRP2070Fab were collected and concentrated to 30 mg/mL using a 100-kDa MWCO Amicon concentrator and then used for crystallisation experiments.
Crystallization and data collection of 5HT1B-BRIL/ERG/SRP2070Fab complex.
5HT1B-BRIL/ ERG/SRP2070Fab were reconstituted into LCP by mixing with host lipids (monoolein:cholesterol=9:1) at a protein:lipid ratio (v:w) of 2:3 using a mixer consisting of two 100 μL gas tight syringes (Hamilton Company). Crystal trays (96 well glass sandwich plates), containing 30 nL LCP sample covered by 800 nL mother liquor per well, were set up using a Gryphon LCP crystallisation robot (Art Robbins Instruments) and then incubated at 20 °C.
Droplets were imaged chronologically by RockImager 1,000 (FORMULATRIX, Bedford, MA). Crystals were obtained from precipitant conditions containing 0.4 M KSCN, 0.1 M NaOAc pH 5.5, and 30% PEG 400. Plate-shaped crystals grew to a maximum size of 30×20×5 μm3 in 3‒4 days, were harvested using MiTeGen MicroMounts (MiTeGen), and flash frozen in liquid nitrogen. X-ray data were collected at the BL32XU beamline at SPring-8 (Japan Synchrotron Radiation Research Institute).
Most crystals diffracted to ~ 3.0 Å resolution (1.0 s exposure, 1.0° oscillation, 10 μm beam attenuated by a 500‒1,200 μm aluminium sheet).
To reduce radiation damage, crystals were exchanged after every 5°–10° of data collection. A complete data set at 3.0 Å resolu- tion was obtained by merging the individual data sets collected from 144 crystals using KAMO28.
The initial structures were solved by molecular replacement as follows: as MR search models, the BRIL/SRP2070 complex (solved in-house) structure was separated into BRIL/Fv region and Fc region, and the receptor region of 5HT1B was clipped from 5HT1B-BRIL structure (PDB ID: 4IAR). The MR search was performed with MOLREP29 using three search models. Rigid-body and restrained refinements were performed with REFMAC530. The ligand was placed in the electron density and the model was corrected with COOT31. The polder ligand omit map 26 was calculated with PHENIX32.
Purification of AT2R-BRIL/s-Ang II/SRP2070Fab.
Residues 1–34 and 347–363 of human AT2R were deleted, and BRIL was inserted between N242 and K246 of AT2R. The HA signal sequence, FLAG tag, and TEV protease recognition site were added to the N-terminus, and TEV protease recognition site and His8 tag were added to the C-terminus of AT2R. The membrane fraction purified from the Sf9 cell pellet was solubilised and incubated (1 h, 4 °C) with solubilisation buffer (50 mM HEPES pH 7.5, 800 mM NaCl, 10% glycerol, 1% DDM, 0.2% CHS, and 15 mM imidazole) supplemented with 100 mg/mL iodoacetamide (Wako Pure Chemical Industries, Ltd.) and 200 μM s-Ang II (Peptide Institute Inc.). After centrifugation (100,000 × g for 30 min, 4 °C), the supernatant was incubated with TALON Superflow Metal Affinity Resin (Clontech, 1–2 mL of resin per 500 mL of original culture volume was used) overnight at 4 °C with gentle agitation. After incubation, the resin was washed with twenty column volumes of wash buffer (50 mM HEPES pH 7.5, 200 mM NaCl, 10% glycerol, 0.1% DDM, 0.02% CHS, 15 mM imidazole, and 200 μM s-Ang II), then eluted by five column volumes of elution buffer (50 mM HEPES pH 7.5, 200 mM NaCl, 10% glycerol, 0.03% DDM, 0.006% CHS, 250 mM imidazole, and 200 μM s-Ang II). The eluted protein sample was concentrated using a 100 kDa MWCO Amicon concentrator, and the buffer was exchanged into wash buffer without imidazole.
The AT2R-BRIL/s-Ang II/SRP2070Fab complex was prepared by mixing AT2R-BRIL/s-Ang II and SRP2070Fab at a molar ratio of 1:1.5 for 1 h on ice.
His-tagged TEV protease was added to the sample and incubated 4 °C overnight to remove the N-terminal FLAG tag and C-terminal His8-tag.
The His8-tagged TEV protease and cleaved C-terminal fragment were removed by incubating with 2 mL of Ni–NTA fast flow resin (GE Healthcare) at 4 °C for 1 h.
AT2R-BRIL/s-Ang II/SRP2070Fab complex was collected as the Ni–NTA column flow-through fraction and concentrated using a 100-kDa MWCO Amicon concentrator. The final complex sample was purified by SEC (Superdex 200 10/300, GE Healthcare). Peak fractions containing AT2R-BRIL/s-Ang II/SRP2070Fab were collected and concentrated to ~ 30 mg/mL using a 100-kDa MWCO Amicon concentrator.
Crystallization and data collection of AT2R-BRIL/s-Ang II/SRP2070Fab complex.
AT2R-BRIL/s- Ang II/SRP2070Fab and host lipids (monoolein:cholesterol=9:1) were mixed at a protein:lipid ratio (v:w) of 2:3 using a mixer consisting of two 100 μL gas tight syringes (Hamilton Company).
Crystal trays (96-well glass sandwich plates), containing 30 nL LCP sample overlaid by 800 nL mother liquor per well, were set up using a NT-8 crystallisation robot (FORMULATRIX), incubated at 20 °C, and imaged chronologically with a RockIm- ager 1,000.
The crystals were obtained from crystallisation conditions containing 50 mM KOAc, 0.1 mM MES pH 6.5, 26–36% PEG 300, 100 μM s-Ang II. Rod-shaped crystals grew to a maximum size of 30 × 5 × 5 μm3 within a week, were harvested using MiTeGen MicroMounts (MiTeGen), and flash frozen in liquid nitrogen. X-ray data were collected at the BL32XU beamline at SPring-8 (Japan Synchrotron Radiation Research Institute).
Most crystals diffracted to ~ 3.5 Å resolution (1.0 s exposure, 1.0° oscillation, 10 μm beam attenuated by a 500–1,200 μm aluminium sheet). To reduce radiation damage, crystals were exchanged after every 5°–10° of data collection.
Finally, a complete data set at 3.4 Å resolution was obtained by merging the individual data sets collected from 118 crystals using the KAMO system28.
The initial structure was determined by molecular replacement with PHASER using BRIL/SRP2070Fv, SRP2070Fv, and the receptor region of AT1R-BRIL (PDB ID: 4ZUD) as search models. Rigid-body and restrained refinements were performed with REFMAC530. The ligand was placed in the electron density and the model was corrected with COOT31. The polder ligand omit map 26 was calculated with PHENIX32.
The detailed crystal packing calculations for 5HT1B-BRIL/ERG/SRP2070Fab and AT2R-BRIL/s-Ang II/SRP- 2070Fab crystal structures were calculated with PISA33 and AREAIMOL in CCP434,35 and shown in Supplementary Table 1S and Supplementary Fig. 4S.
Structural figures were prepared using the CCP4mg molecular-graphics software36 except for Fig. 4C,D. Figure 4C,D were prepared using CueMol.
